When you pick up a prescription at the pharmacy, you might see two bottles: one with a familiar brand name, another with a plain label and a much lower price. You might wonder-does the cheaper version really work the same? The answer lies in bioequivalence testing, a process that’s been quietly ensuring millions of people get safe, effective medications every day without paying brand-name prices.
What Bioequivalence Testing Actually Measures
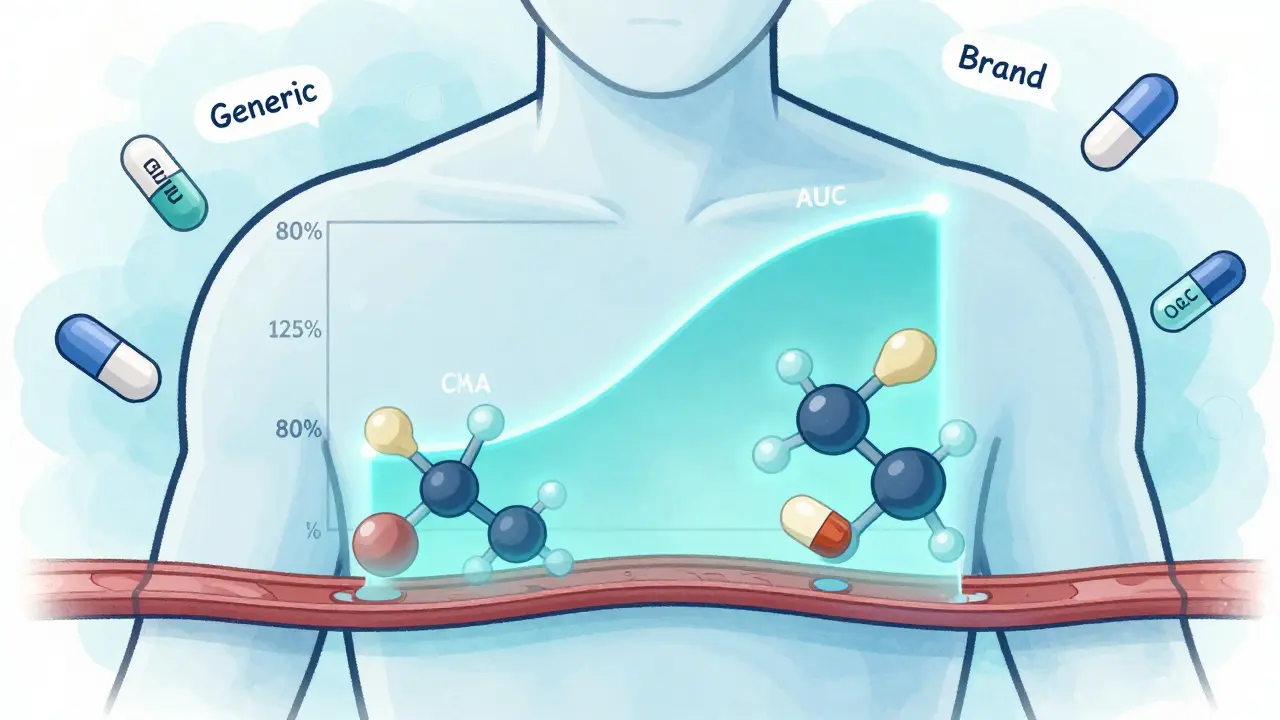
Bioequivalence testing doesn’t check if a generic drug cures the same disease as the brand-name version. That’s already proven. Instead, it asks one precise question: Does the generic drug get into your bloodstream at the same rate and in the same amount as the brand?
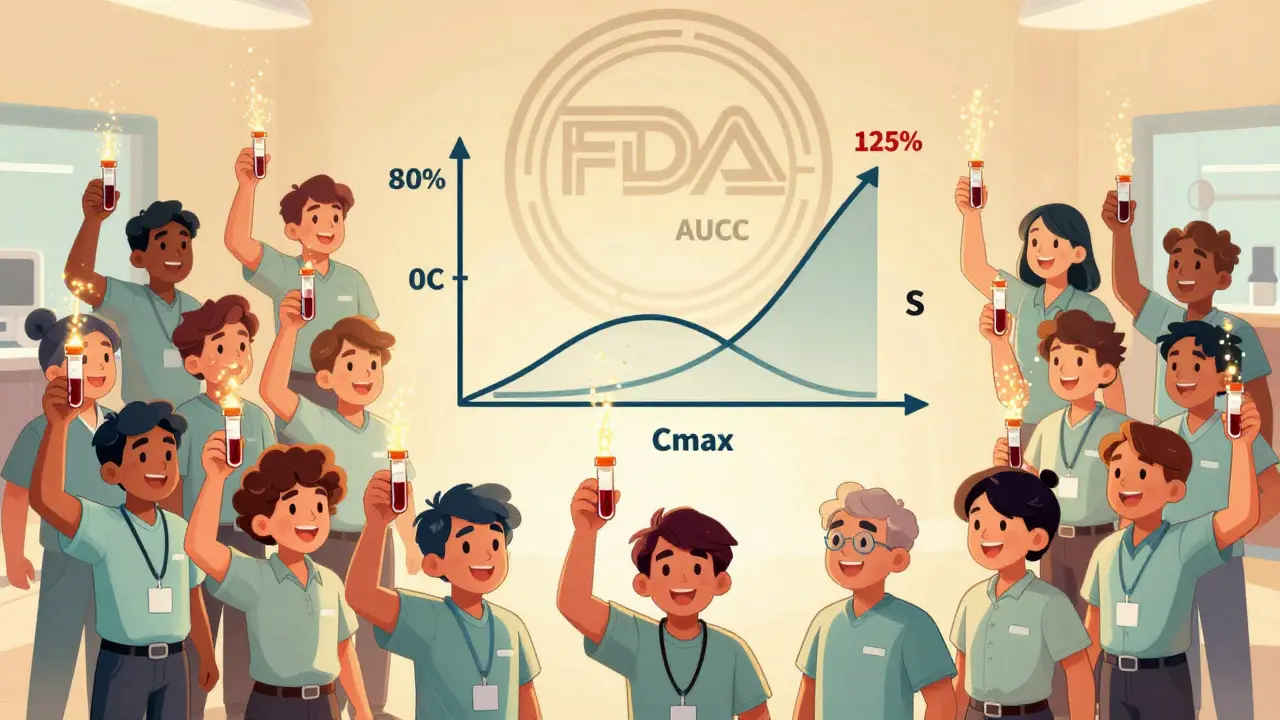
The FDA defines bioequivalence as the absence of a significant difference in how quickly and how much of the active ingredient reaches your bloodstream. For most oral pills, that means measuring two key numbers: AUC (area under the curve) and Cmax (peak concentration). AUC tells you how much of the drug your body absorbs overall. Cmax tells you how fast it gets there. If both fall within 80% to 125% of the brand-name drug’s numbers, the FDA considers them bioequivalent.
These numbers aren’t guesses. They come from real human studies. Typically, 24 to 36 healthy volunteers take both the generic and brand versions in a crossover design-meaning they take one, wait a few days, then take the other. Blood is drawn over hours to track concentration levels. The data is analyzed statistically. If the 90% confidence interval for the ratio of generic to brand stays between 80% and 125% for both AUC and Cmax, the drug passes.
Why This Matters More Than You Think
Many people assume that if a drug looks different-different color, shape, or filler-it must work differently. But inactive ingredients don’t affect how the active drug is absorbed. That’s why generic manufacturers are allowed to change those. The active ingredient? Same molecule. Same dose. Same strength.
Take lisinopril, a common blood pressure pill. The brand-name version, Prinivil, costs around $150 for a 30-day supply. The generic? About $4. Both contain the exact same chemical compound. Bioequivalence testing confirmed they release the same amount of lisinopril into the blood at the same pace. That’s why doctors prescribe generics without hesitation. In fact, over 90% of prescriptions in the U.S. are filled with generics.
The savings aren’t small. In 2020 alone, generics saved the U.S. healthcare system $313 billion. That’s money that goes back into hospitals, research, and patient care. Without bioequivalence testing, those savings wouldn’t exist. Generic manufacturers wouldn’t be able to skip expensive clinical trials because they’re relying on the brand’s proven safety and effectiveness. All they need to prove is that their version behaves the same way in the body.
How It’s Different From Brand-Name Drug Approval
Brand-name drugs go through years of testing. Phase I trials check safety in small groups. Phase II and III involve hundreds or thousands of patients to prove effectiveness and monitor side effects. It can cost over $2 billion and take a decade.
Generic drugs don’t repeat that. They start with a simple question: Is this version absorbed the same way? That’s it. The FDA doesn’t require new safety or efficacy data because the brand’s data already exists. The generic only needs to prove it delivers the same amount of drug to the same place at the same speed.
This isn’t cutting corners. It’s smart science. If a drug has been used safely by millions, why test it again from scratch? Bioequivalence testing is the bridge between innovation and accessibility. It lets new manufacturers enter the market without repeating what’s already known.
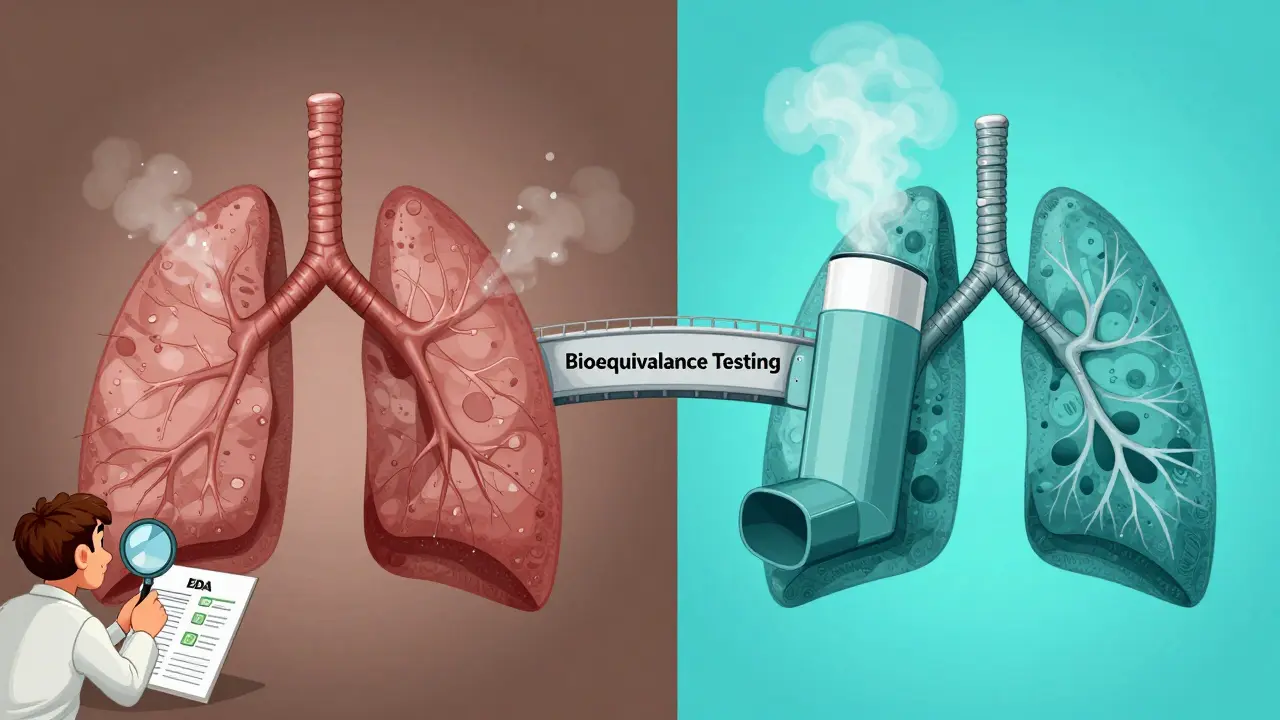
Where Bioequivalence Testing Falls Short
Not all drugs are created equal. For most pills, bioequivalence testing works perfectly. But for some complex products, measuring blood levels doesn’t tell the whole story.
Take inhalers. The drug needs to reach your lungs, not just show up in your bloodstream. A generic inhaler might have the same active ingredient and dose, but if the spray pattern, particle size, or delivery mechanism differs slightly, it might not deliver the drug where it’s needed. That’s why the FDA requires additional testing for inhalers-sometimes clinical endpoint studies that measure lung function or symptom control, not just blood levels.
The same goes for topical creams. A generic hydrocortisone cream might have the same concentration of steroid, but if the base (the cream’s texture or ingredients) changes, it might not penetrate the skin the same way. The FDA now requires in vivo studies for certain topical products to make sure the drug actually reaches the affected area.
Narrow therapeutic index drugs are another challenge. These are medications where even a small difference in blood level can cause harm or reduce effectiveness. Think warfarin, lithium, or certain epilepsy drugs. For these, the FDA sometimes tightens the bioequivalence range to 90-111% instead of 80-125%. That extra precision ensures safety.
What the Data Says About Real-World Use
Do people notice a difference? According to a 2022 Consumer Reports survey of 1,200 users, 87% reported no difference between their generic and brand-name drugs. Nine percent said the generic worked better. Only 4% said it worked less effectively.
On Reddit’s r/pharmacy community, a 2023 thread with over 1,400 comments showed that 78% of people who shared experiences saw no change. Most complaints weren’t about effectiveness-they were about side effects from inactive ingredients. One person said their generic version of levothyroxine gave them stomach upset because of a new filler. Another said their generic ADHD pill made them feel jittery. These aren’t bioequivalence failures. They’re reactions to dyes, binders, or coatings that changed. Switching back to the brand-or trying a different generic-often fixes it.
Still, myths persist. A 2021 study found 32% of patients believed generics were less effective. Some think they “take longer to work.” Others worry they’re “weaker.” These beliefs aren’t based on science. They’re based on fear, cost stigma, or anecdotal stories. The data doesn’t support them.
The Global Standard and Future of Testing
Bioequivalence standards aren’t just an American thing. The European Medicines Agency, Health Canada, Japan’s PMDA, and others follow similar rules. The International Council for Harmonisation (ICH) helped align these standards globally. That means a generic approved in Australia, the U.S., or Germany is held to the same scientific bar.
The future is getting smarter. The FDA is exploring computer modeling to predict how a drug will behave in the body-called physiologically based pharmacokinetic (PBPK) modeling. This could reduce the number of human studies needed, especially for complex drugs. Imagine running simulations to test dozens of formulations before ever giving a pill to a volunteer. It’s already being used for some inhalers and injectables.
Meanwhile, the Generic Drug User Fee Amendments (GDUFA) program has cut approval times from over two years to under a year, without lowering standards. In 2022, 95% of generic applications were reviewed on time.
What You Should Know Before Taking a Generic
If your doctor prescribes a generic, you can feel confident. The system works. Bioequivalence testing is rigorous, science-based, and backed by decades of real-world use.
But if you notice a change-new side effects, less effectiveness, or unusual symptoms-talk to your pharmacist. It might be the filler, not the drug. Ask if there’s another generic made by a different company. Not all generics use the same inactive ingredients.
For complex drugs-like inhalers, patches, or injectables-ask your doctor if bioequivalence testing was done with additional methods. Don’t assume all generics are the same. Some need extra proof.
And remember: the price difference isn’t a trick. It’s the result of smart regulation. Bioequivalence testing lets competition work. It keeps prices low without sacrificing safety. That’s why over 20,000 generic drugs are approved in the U.S. and why 90% of prescriptions use them.
The bottom line? Bioequivalence testing doesn’t just prove a generic drug works. It proves it works the same way as the brand-down to the last molecule. And that’s why it’s one of the most successful public health policies in modern medicine.
Does bioequivalence mean a generic drug is exactly the same as the brand?
No, bioequivalence doesn’t mean the drugs are identical in every way. The active ingredient, strength, and dosage form must be the same, but generics can differ in color, shape, flavor, and inactive ingredients like fillers or dyes. What matters is that the active drug enters your bloodstream at the same rate and amount. That’s what bioequivalence testing confirms.
Are generics less effective for serious conditions like heart disease or epilepsy?
For the vast majority of patients, generics work just as well. But for drugs with a narrow therapeutic index-like warfarin, lithium, or phenytoin-doctors may prefer to stick with one brand or generic to avoid even small variations. The FDA applies stricter bioequivalence standards (90-111%) for these drugs. If you’re on one of these, talk to your doctor before switching. But don’t assume generics are unsafe-they’re still held to high standards.
Why do some people say generics don’t work for them?
Most of the time, it’s not the active drug. It’s the inactive ingredients. Some people are sensitive to dyes, gluten, or fillers used in a particular generic version. Switching to a different generic made by another manufacturer often fixes the issue. If you notice new side effects after switching, talk to your pharmacist-they can help you find a version with a different formulation.
Is bioequivalence testing the same around the world?
Yes, the core standards are aligned globally through the ICH. The U.S. FDA, EMA in Europe, Health Canada, and Japan’s PMDA all require the 80-125% range for AUC and Cmax for most oral drugs. Some regions may add extra requirements for complex products like inhalers or topical creams, but the foundation is the same. A generic approved in Australia meets the same scientific bar as one approved in the U.S.
Can I trust a generic drug made overseas?
Yes. The FDA inspects over 1,200 generic drug manufacturing facilities each year-half of them outside the U.S. All generics sold in the U.S. must meet the same quality standards, no matter where they’re made. The FDA doesn’t allow lower standards just because a drug is imported. If it’s on the market, it passed inspection and bioequivalence testing.


Yeah but have you ever tried switching generics and ended up feeling like a zombie for a week? My cousin swore her epilepsy meds went from ‘life-saving’ to ‘life-sucking’ after a pharmacy change. No one listens until you’re barely functional.