When doctors talk about blocking the hormone aldosterone, eplerenone is a selective mineralocorticoid receptor antagonist developed to treat high blood pressure and certain forms of heart failure often pops up. It emerged from a need for a cleaner alternative to older drugs that caused unwanted side effects.
Quick Takeaways
- Eplerenone blocks aldosterone more selectively than spironolactone, reducing hormone‑related side effects.
- It was approved by the FDA in 2002 after the pivotal EPHESUS trial showed mortality benefits for heart‑failure patients.
- Pharmacokinetic profile: oral bioavailability ~70%, half‑life ~4‑6 hours, processed mainly by CYP3A4.
- Common uses include managing hypertension and post‑myocardial‑infarction heart failure with reduced ejection fraction.
- Future research looks at combining eplerenone with newer SGLT2 inhibitors for synergistic heart‑failure therapy.
Chemical Roots and Early Research
The story starts in the late 1980s when scientists at Merck were hunting for a molecule that could inhibit the mineralocorticoid receptor (MR) without touching other steroid receptors. The lead compound, initially called "SC‑66128," showed promising in‑vitro binding affinity for the MR. After a series of structure‑activity relationship studies, the team introduced a bulky 9‑hydroxy group, creating the final structure of eplerenone.
This tweak gave the drug a high selectivity ratio: it bound the MR about 20‑times more strongly than the androgen receptor, a crucial improvement over spironolactone, which interacts with multiple steroid receptors and can cause gynecomastia in men.
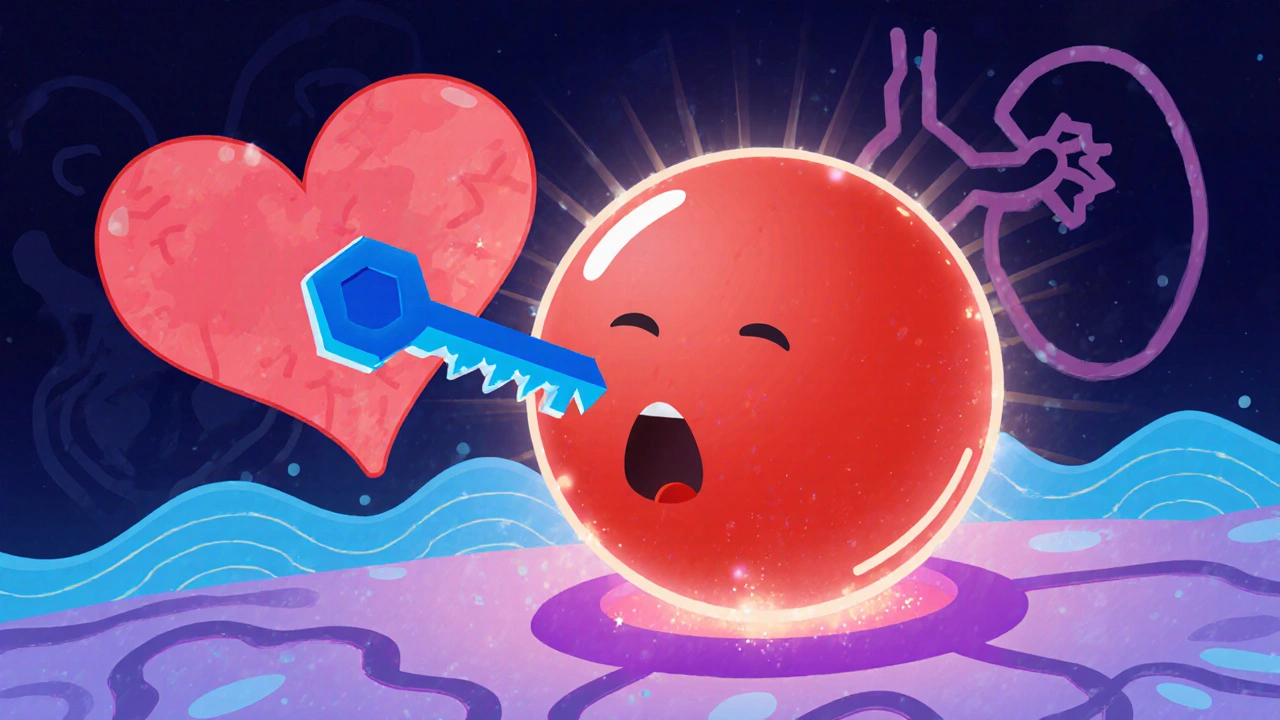
Mechanism of Action: How It Blocks Aldosterone
Aldosterone is a hormone that tells the kidneys to retain sodium and excrete potassium. In excess, it drives fluid overload, high blood pressure, and heart‑muscle remodeling. Aldosterone is a mineralocorticoid hormone produced by the adrenal cortex that regulates sodium and water balance binds to the mineralocorticoid receptor (Mineralocorticoid Receptor is a nuclear receptor that mediates the effects of aldosterone on gene transcription in kidney and heart cells). Eplerenone competes for the same binding pocket, preventing aldosterone from activating the receptor.
Because the drug does not significantly antagonize androgen or progesterone receptors, patients experience fewer hormonal side effects. The downstream effect is reduced sodium reabsorption, lower plasma volume, and diminished cardiac fibrosis.
Drug Development Timeline
- 1990‑1995: Discovery and pre‑clinical testing in rodent models demonstrated robust MR blockade and lowered blood pressure.
- 1996‑1999: Phase I studies confirmed safety and a dose‑dependent plasma concentration profile.
- 2000: The pivotal EPHESUS (Eplerenone Post‑Acute Myocardial Infarction Study) began, enrolling over 6,500 patients with left‑ventricular dysfunction.
- 2002: FDA Approval was granted for eplerenone tablets (Inspra) to treat hypertension and post‑myocardial‑infarction heart failure.
- 2005‑2010: Post‑marketing surveillance confirmed a lower incidence of gynecomastia compared with spironolactone.
- 2020‑2024: New trials explored eplerenone in combination with SGLT2 inhibitors and in diabetic kidney disease.
Clinical Trial Milestones
The EPHESUS trial remains the cornerstone of eplerenone’s reputation. Over a median follow‑up of 2.6 years, patients receiving eplerenone had a 15 % relative reduction in all‑cause mortality and a 13 % reduction in cardiovascular death or hospitalization for heart failure compared with placebo.
Another key study, the EMPHASIS‑HF (Eplerenone in Mild Patients Hospitalization And Survival Study in Heart Failure), focused on patients with less severe left‑ventricular dysfunction (ejection fraction ≤35 %). That trial showed a 24 % drop in the combined endpoint of cardiovascular death or heart‑failure hospitalization.
These outcomes cemented eplerenone’s role in guideline‑directed therapy for heart failure with reduced ejection fraction (HFrEF).
Pharmacokinetics and Dosing
Eplerenone is administered orally, with an average bioavailability of about 70 %. Food can slightly delay absorption but does not affect overall exposure. The drug is metabolized primarily by the cytochrome P450 enzyme CYP3A4; strong inhibitors like ketoconazole can raise plasma levels by up to 2‑fold, while inducers like rifampin can cut exposure by half.
Typical dosing for hypertension starts at 25 mg once daily, titrated to 50 mg as needed. For heart‑failure patients, the usual regimen is 25 mg daily, increased to 50 mg after four weeks if tolerated.
Because the half‑life is relatively short (4‑6 hours), steady‑state concentrations are reached within 2‑3 days, allowing clinicians to adjust doses quickly.
Safety Profile Compared to Spironolactone
Spironolactone, the older MR antagonist, is effective but notorious for anti‑androgenic side effects. To illustrate the difference, see the comparison table below.
| Aspect | Eplerenone | Spironolactone |
|---|---|---|
| Receptor Selectivity | High (MR‑specific) | Low (binds androgen & progesterone receptors) |
| Risk of Gynecomastia | ~1‑2 % | ~10‑15 % |
| Potassium‑sparing Effect | Comparable | Comparable |
| Typical Dose (HF) | 25‑50 mg daily | 25‑50 mg daily |
| Major Drug Interactions | CYP3A4 inhibitors/inducers | Less CYP‑dependent |
Both drugs can cause hyperkalaemia, especially when combined with ACE inhibitors or ARBs. However, the lower incidence of hormonal side effects makes eplerenone the preferred choice for many clinicians, particularly in male patients.

Current Uses and Guideline Recommendations
Guidelines from the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) list eplerenone as a Class I recommendation for patients with HFrEF who have an ejection fraction ≤35 % and are already on ACE‑I/ARB and beta‑blocker therapy.
Beyond heart failure, eplerenone is approved for stage 1 or 2 hypertension that does not respond adequately to first‑line agents. It is sometimes used off‑label for primary aldosteronism, though specialist consultation is advised.
Future Directions and Ongoing Research
Researchers are testing whether adding eplerenone to SGLT2 inhibitors like dapagliflozin yields additive mortality reductions. Early phase II data suggest improved renal outcomes, but larger phase III trials are still enrolling.
Another frontier is the use of eplerenone in chronic kidney disease (CKD) patients with resistant hypertension. A 2023 multicenter trial reported a 12 % slower decline in estimated glomerular filtration rate (eGFR) over three years compared with standard care.
Finally, formulation scientists are developing extended‑release tablets to smooth out peak‑trough fluctuations, potentially reducing the need for twice‑daily dosing in patients with erratic adherence.
Key Points for Patients and Clinicians
- Check for drug interactions, especially CYP3A4 modulators.
- Monitor serum potassium and renal function within the first month of therapy.
- Educate patients about the low risk of hormonal side effects, but still watch for breast tenderness.
- Adjust dose slowly in elderly patients or those with moderate hepatic impairment.
What is the main difference between eplerenone and spironolactone?
Eplerenone is far more selective for the mineralocorticoid receptor, which means it causes far fewer anti‑androgenic side effects like gynecomastia compared with spironolactone.
Can eplerenone be used in patients with kidney disease?
Yes, but kidney function must be closely monitored because the drug can raise potassium levels. Dose reductions are common in stage 3‑4 CKD.
How long does it take to see blood‑pressure effects?
Most patients notice a modest drop in systolic pressure within 1‑2 weeks, with the full effect reached after about 4‑6 weeks of steady dosing.
Is eplerenone safe to take with ACE inhibitors?
Combining eplerenone with ACE inhibitors is standard in heart‑failure therapy, but clinicians must watch potassium and renal labs closely, especially during the first month.
What should I do if I miss a dose?
Take the missed dose as soon as you remember if it’s within 12 hours; otherwise, skip it and resume the regular schedule. Do not double‑dose.


If you think the novelty of eplerenone lies in its brand name, you're missing the real pharmacological nuance. The drug's selectivity for the mineralocorticoid receptor is a textbook case of rational design, not a marketing gimmick. Its development history illustrates how medicinal chemistry can refine a scaffold to dodge androgenic side effects, a point many clinicians overlook. Moreover, the reliance on CYP3A4 for metabolism makes drug‑drug interactions a pivotal consideration in polypharmacy. In practice, the modest half‑life translates to steady‑state achievement within days, allowing dose titration without excessive delay.