When a patient walks into your office with a prescription for a brand-name drug, do you ever wonder if a cheaper generic could work just as well? You’re not alone. Despite generics making up 90% of all prescriptions filled in the U.S., many doctors still hesitate to switch patients - not because they doubt the science, but because they lack clear, practical tools to explain it.
The truth is, generic drugs aren’t just cheaper. They’re scientifically identical in how they work. The U.S. Food and Drug Administration (FDA) is the federal agency responsible for approving and monitoring generic medications to ensure they meet strict standards for safety, strength, and effectiveness. Every generic must prove it delivers the same active ingredient at the same rate and extent as the brand-name version. That’s not a guess - it’s a requirement backed by clinical studies in healthy volunteers. The FDA requires bioequivalence within an 80% to 125% range, meaning the body absorbs the generic drug just as reliably as the original.
Why Doctors Still Hold Back
It’s not about safety. In 2022, the FDA analyzed over 24,000 adverse event reports for generic and brand-name drugs. The numbers were nearly identical: 12,467 for generics, 11,832 for brands. So why do some physicians still default to brand names?
Two big reasons: confusion and lack of time.
Many doctors aren’t trained on the finer points of bioequivalence. They’ve heard myths - that generics are made in lower-quality factories, or that they don’t work for elderly patients or those with complex conditions. A 2023 survey found 61% of physicians were unsure what "authorized generics" are - brand-name drugs sold under a generic label, identical in every way except packaging and price.
And then there’s the clock. The average primary care visit lasts 15 minutes. You’ve got to diagnose, explain, answer questions, and document. Finding a PDF on your desktop or hunting through a website isn’t practical. One family doctor in Nebraska told the Journal of the American Board of Family Medicine that she increased her generic prescribing from 62% to 89% - not because she changed her mind, but because she finally had a visual tool: the FDA’s infographic comparing manufacturing standards side-by-side. "It helped me show patients the truth," she said. "Not just tell it. Show it."
What the FDA Offers - And How to Use It
The FDA doesn’t just publish guidelines. It builds tools designed for real clinics. Their Generic Drugs Stakeholder Toolkit is a free, downloadable collection of ready-to-use materials for prescribers, including social media templates, patient handouts, and visual aids.
Here’s what actually works in practice:
- Prescriber Flyers (142 KB PDF) - One-page, double-sided references that fit in office literature racks. Version 2 (March 2022) includes QR codes linking to Spanish-language resources, addressing health disparities. Hispanic patients are 42% more likely to question generic quality - this tool helps bridge that gap.
- Infographic: "What Makes a Generic the Same?" - A 431 KB visual that breaks down the 80-125% bioequivalence range, manufacturing oversight, and how the FDA tests for consistency. It’s not jargon-heavy. It’s drawn like a flowchart a 6th grader could understand.
- Generic Drugs and Health Equity Handout - This one’s critical. It shows data: patients earning under $25,000/year are 3.7 times more likely to stop taking essential meds because of cost. That’s not a statistic - it’s your next patient.
These aren’t just documents. They’re conversation starters. A 2022 study found that physicians who used these materials were 2.3 times more likely to bring up cost with patients. That’s huge. When patients hear "this generic will save you $262.50 a month," and see a simple chart showing identical absorption curves, they’re far more likely to agree.

Where the System Falls Short
Here’s the problem: these tools aren’t built into your workflow.
A 2023 KLAS Research report found only 37% of major electronic health record (EHR) systems - like Epic or Cerner - integrate FDA generic education pop-ups. That means you have to leave your screen, open a browser, find a PDF, print it, or email it. Most don’t.
One doctor on Reddit, DrJenkins_MD, put it bluntly: "I need this info in my Epic alert box, not as a PDF I have to hunt for."
That’s why Kaiser Permanente’s 2021 fix worked so well. They embedded FDA-approved generic prescribing tips directly into their Epic system. When a doctor selected a brand-name statin, a small pop-up appeared: "Generic available. Saves patient $210/month. Therapeutic equivalence confirmed." Within six months, brand-name prescribing dropped by 18.7%.
That’s not magic. That’s design.

What You Can Do Today
You don’t need a hospital-wide system change to start using these resources. Here’s how to make it work in your clinic:
- Download the FDA Prescriber Flyer - Print it. Tape it to your computer monitor. Put it in your exam room. Keep one in your pocket.
- Use the infographic in consultations - Don’t say "it’s the same." Show the patient the side-by-side comparison. Point to the 80-125% range. Say: "This is what the FDA requires. Your body processes it the same way."
- Start with one patient - Pick a patient on a $300/month brand-name drug. Switch them to the generic. Calculate the savings: $262.50/month. Tell them. They’ll remember it.
- Ask your EHR vendor - If your system doesn’t have generic prompts, ask if they can integrate FDA data. It’s not hard. The FDA launched an API in July 2023 specifically for this.
Dr. Aaron Kesselheim from Harvard put it simply: "The most effective education combines science with savings. Show the data. Show the cost. Let the patient choose - but give them the truth first."
What’s Changing Now
The landscape is shifting fast. In 2024, Medicare Part D proposed new rules that will financially reward insurance plans that help prescribers learn about therapeutic alternatives. That means more funding, more training, and more pressure to adopt.
IBM Watson Health tested AI-generated recommendations tailored to individual patient concerns - like anxiety about switching or past bad experiences. In a trial with 120 doctors, acceptance rates jumped by 29 percentage points. That’s not science fiction. It’s coming.
And yet, the biggest barrier isn’t technology. It’s habit. We’ve been taught to trust the brand name. The packaging. The name on the bottle. But the pill inside? That’s what matters. And the FDA has spent over a decade proving it’s identical.
Every time you choose a generic - not because it’s cheaper, but because it’s just as good - you’re not just saving money. You’re saving adherence. You’re saving lives. And you’re doing it with evidence, not guesswork.
Are generic drugs really as effective as brand-name drugs?
Yes. Every generic drug approved by the FDA must prove it delivers the same active ingredient, in the same amount, and at the same rate as the brand-name version. This is called bioequivalence. The FDA requires testing in healthy volunteers to show absorption falls within an 80%-125% range - meaning the body processes it identically. Over 90% of prescriptions in the U.S. are for generics, and studies show no difference in clinical outcomes.
Why do some patients refuse generic medications?
Many patients believe generics are inferior because of marketing, packaging, or past experiences. Some think "cheaper = less effective." Others worry about manufacturing quality - especially if they’ve heard rumors about overseas production. The FDA’s infographic showing side-by-side manufacturing standards helps address these fears. Patients with lower incomes are especially likely to discontinue meds due to cost, so explaining savings is key.
Can generics be used for complex medications like inhalers or biologics?
For simple oral medications - like statins, antibiotics, or blood pressure pills - yes, without question. For complex drugs like inhalers, injectables, or topical creams, equivalence is harder to prove. These are called "complex generics" and require more testing. Biosimilars (a type of generic for biologic drugs) are still evolving. Only 42% of prescribers use available education on them, according to a 2023 FDA report. Always check the specific drug class before switching.
What’s the difference between generic and authorized generics?
An authorized generic is made by the original brand-name company and sold under a generic label. It’s identical in every way - same factory, same formula, same packaging (except for the name). Yet, 61% of physicians don’t know this exists. Authorized generics are often cheaper than brand-name versions but more expensive than standard generics. They’re a great option if the patient is skeptical about generics.
How can I integrate generic education into my busy clinic?
Start small. Print the FDA Prescriber Flyer and keep it near your computer. Use the infographic during patient visits. Ask your EHR vendor if they can add FDA generic alerts. Train your staff to mention cost savings during check-in. One study found just 22 minutes of education was enough to change prescribing habits. You don’t need a program - just a habit.
Do insurance companies force doctors to prescribe generics?
Insurance plans often require patients to try generics first (called "step therapy"), but they can’t force you to prescribe. However, if you prescribe a brand-name drug without a medical reason, the patient may face higher out-of-pocket costs. Many states now have laws requiring pharmacists to substitute generics unless you write "Do Not Substitute" on the script. Education helps you make informed decisions - not just react to pressure.
Is there data showing that prescribing generics improves patient outcomes?
Yes. The American College of Physicians found that cost is a leading cause of medication non-adherence - affecting 20-30% of new prescriptions. When patients save hundreds per month, they’re far more likely to take their meds consistently. A 2021 study in Health Affairs showed patients switched to generics were 17% more likely to stay on therapy long-term. Better adherence means fewer hospital visits, fewer complications, and better health outcomes.
Prescribing generics isn’t about cutting corners. It’s about cutting waste - waste in cost, waste in health, waste in trust. The science is solid. The tools are free. The time is now.

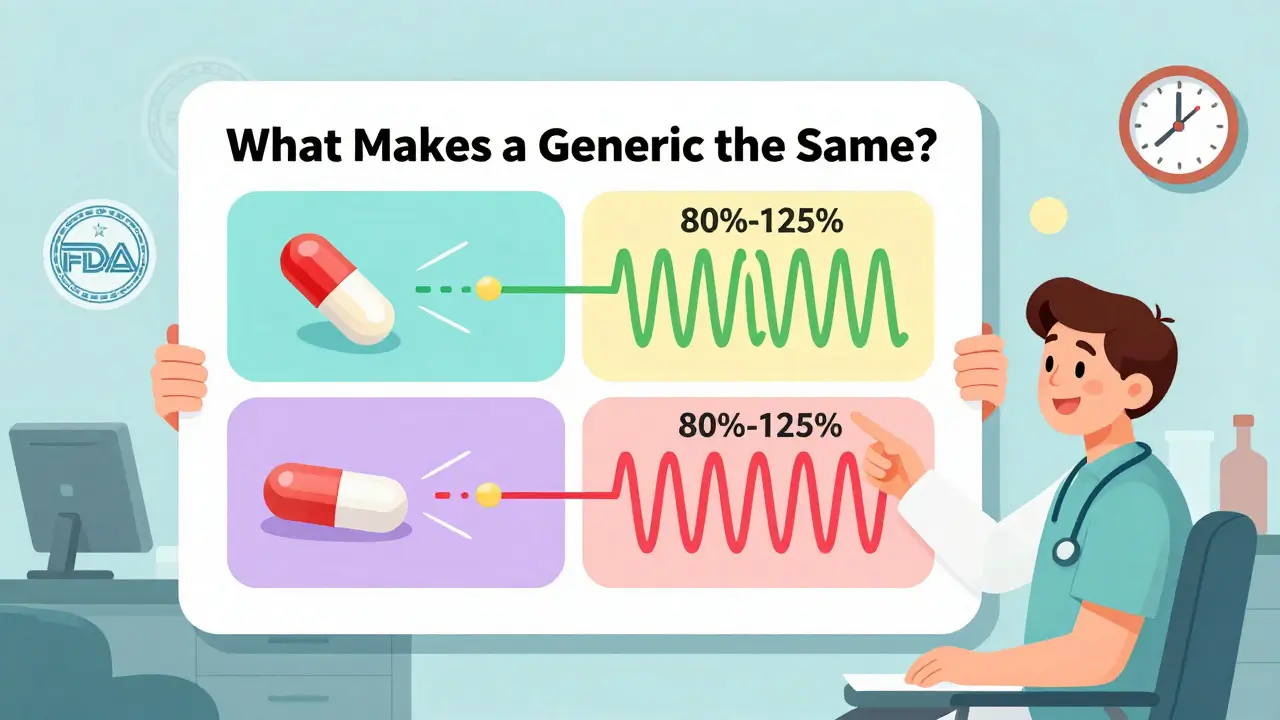

So i just read this whole thing and honestly? I’m shocked. I’ve been prescribing generics for years but never knew about the FDA’s infographic. I printed it out and taped it to my monitor. My PA just asked me what the weird drawing was. Told her. She’s gonna start using it too. Game changer. Seriously.