Tag: bioequivalence
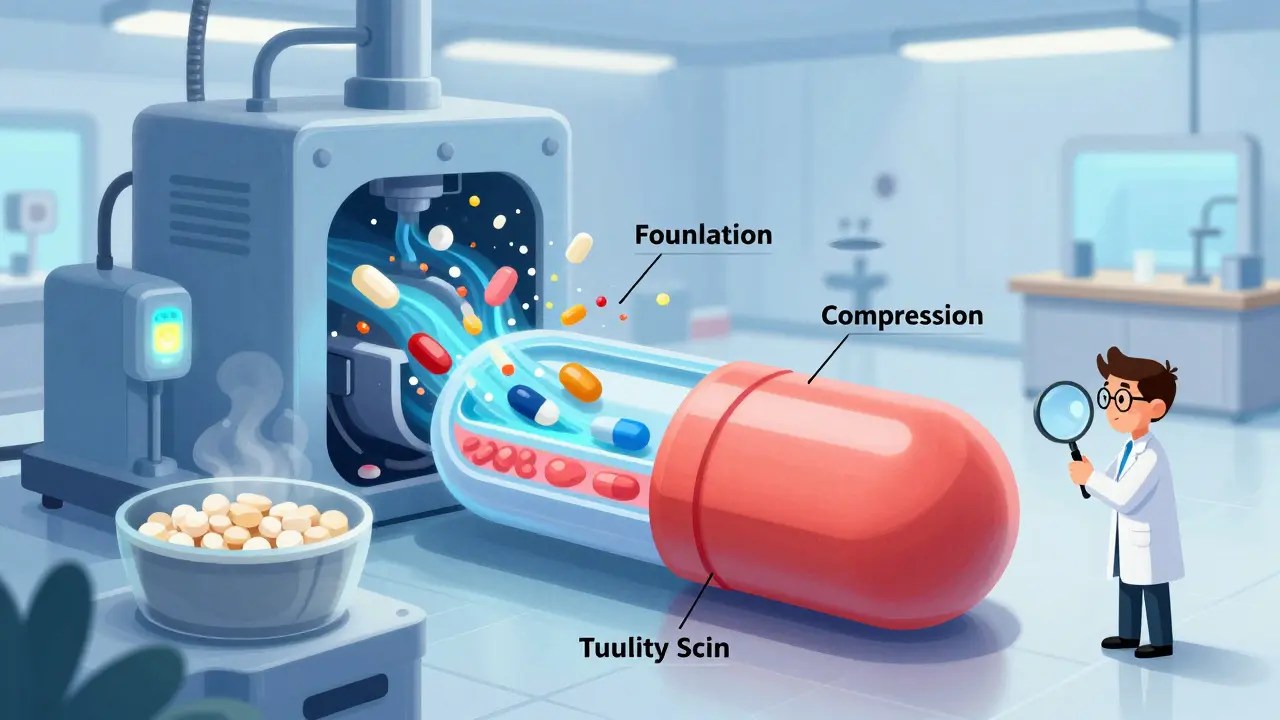
How Generic Drugs Are Made: The Manufacturing Process Explained
Posted by Desmond Carrington on 7/02/26
Generic drugs save billions by offering the same medicine as brand-name drugs at a fraction of the cost. Learn how they're made, tested, and approved under strict FDA rules to ensure safety and effectiveness.
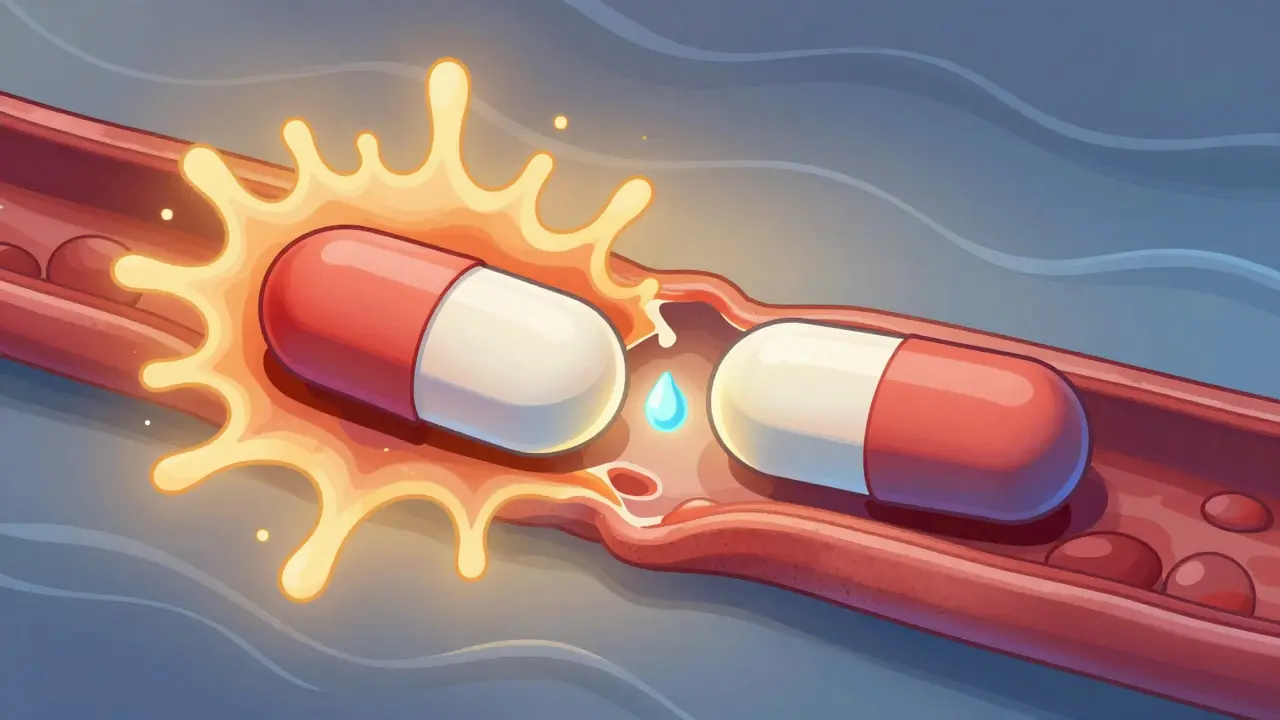
Modified-Release Formulations: What You Need to Know About Bioequivalence Rules
Posted by Desmond Carrington on 30/01/26
Modified-release formulations require specialized bioequivalence testing to ensure generics behave like brand-name drugs. Learn how regulators assess release profiles, partial AUC, alcohol interactions, and why these drugs are harder to copy.

How the FDA Ensures Generic Drugs Work the Same as Brands
Posted by Desmond Carrington on 12/12/25
The FDA ensures generic drugs work the same as brand-name medications through strict bioequivalence testing, identical active ingredients, and rigorous manufacturing standards. Over 90% of U.S. prescriptions are filled with generics that save billions annually.

