Tag: generic drugs
Medical Society Guidelines on Generic Drug Use: What Doctors Really Think
Posted by Desmond Carrington on 18/02/26
Medical societies have clear, sometimes conflicting, positions on generic drug substitution. While most generics are safe, drugs with narrow therapeutic indices require caution. Learn which ones to avoid swapping and why doctors push back.
Prescriber Education Resources: Guides for Doctors on Generics
Posted by Desmond Carrington on 7/02/26
Doctors need clear, practical tools to confidently prescribe generic medications. Learn how FDA resources help bridge the gap between science and practice - and why 90% of prescriptions are already generics.
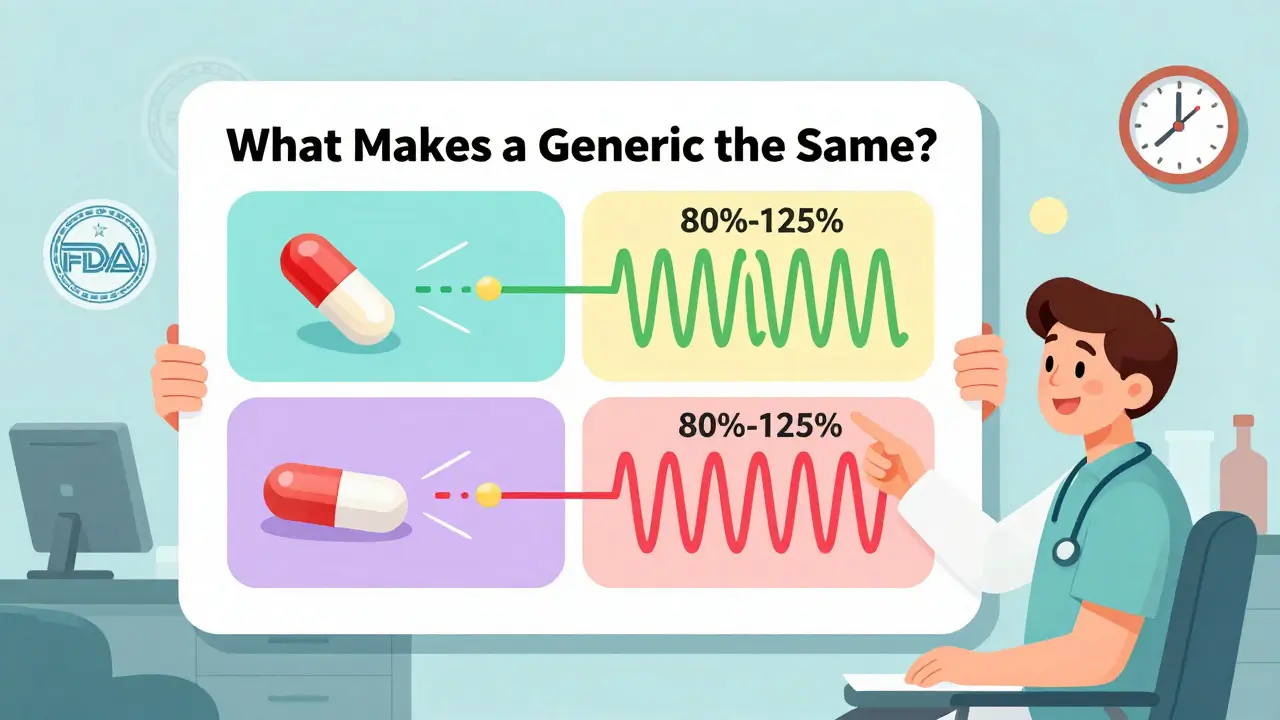
Bioequivalence Testing for Generic Drugs: What It Actually Proves
Posted by Desmond Carrington on 2/02/26
Bioequivalence testing proves generic drugs deliver the same active ingredient at the same rate and amount as brand-name versions. It's the science behind 90% of prescriptions in the U.S. and saves billions annually.
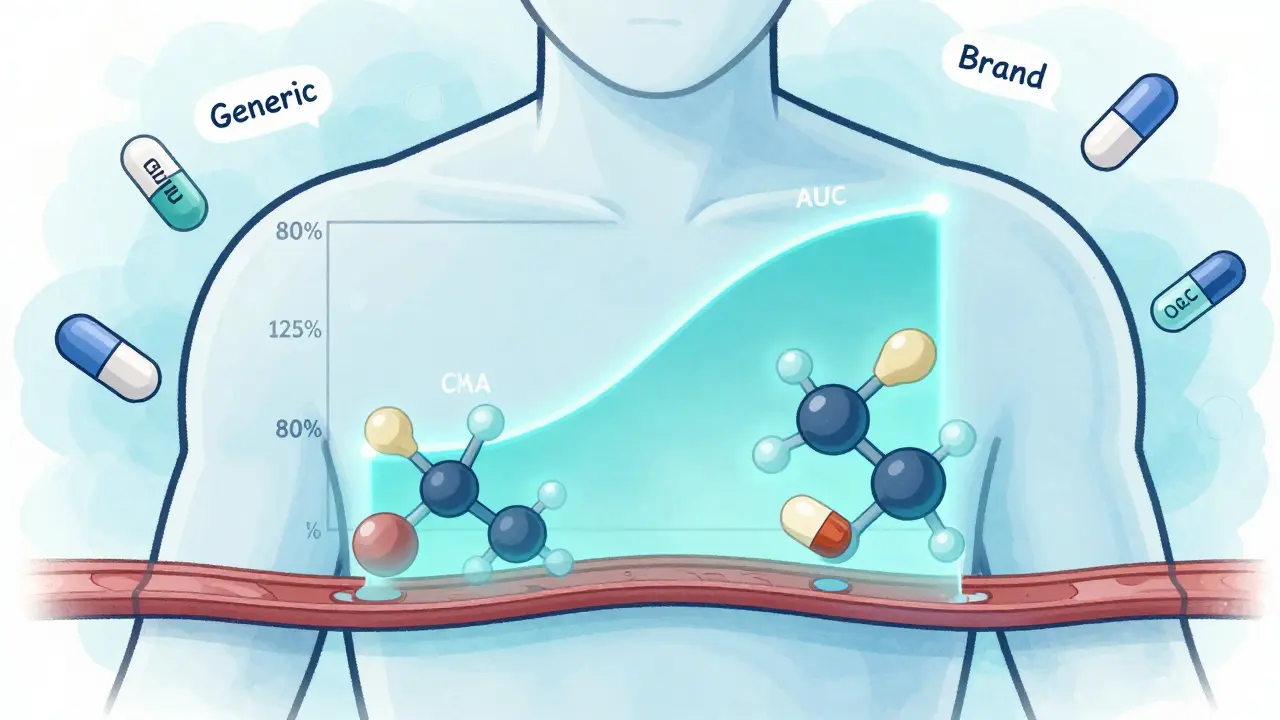
Modified-Release Formulations: What You Need to Know About Bioequivalence Rules
Posted by Desmond Carrington on 30/01/26
Modified-release formulations require specialized bioequivalence testing to ensure generics behave like brand-name drugs. Learn how regulators assess release profiles, partial AUC, alcohol interactions, and why these drugs are harder to copy.

How the FDA Ensures Generic Drugs Work the Same as Brands
Posted by Desmond Carrington on 12/12/25
The FDA ensures generic drugs work the same as brand-name medications through strict bioequivalence testing, identical active ingredients, and rigorous manufacturing standards. Over 90% of U.S. prescriptions are filled with generics that save billions annually.

Workers' Compensation and Generic Substitution: What You Need to Know in 2025
Posted by Desmond Carrington on 27/11/25
Generic substitution is now standard in workers' compensation, cutting drug costs by up to 80% without sacrificing effectiveness. Learn how it works, why it's mandatory in most states, and what you need to know in 2025.

Medication Costs: How Coupons, Generics, and Prior Authorizations Affect Your Pocket in 2025
Posted by Desmond Carrington on 22/11/25
Learn how coupons, generics, and prior authorizations impact your medication costs in 2025. Discover real ways to save hundreds on prescriptions with Medicare's new price rules and smart shopping tips.

Switching from Brand to Generic Drugs: What to Expect
Posted by Desmond Carrington on 10/11/25
Switching from brand to generic drugs can save you hundreds a year-but not all switches are safe. Learn when it's fine, when to be cautious, and what to do if you feel different after switching.

