Archive: 2026/01
Modified-Release Formulations: What You Need to Know About Bioequivalence Rules
Posted by Desmond Carrington on 30/01/26
Modified-release formulations require specialized bioequivalence testing to ensure generics behave like brand-name drugs. Learn how regulators assess release profiles, partial AUC, alcohol interactions, and why these drugs are harder to copy.
Opioid Reactions: Itching vs. Allergy and What to Do
Posted by Desmond Carrington on 29/01/26
Itching from opioids is rarely a true allergy - it's usually a histamine reaction. Learn how to tell the difference, what to do when it happens, and which pain meds won't make you scratch.

How to Talk to Your Doctor About New Drug Safety Alerts
Posted by Desmond Carrington on 28/01/26
Learn how to effectively discuss FDA drug safety alerts with your doctor-without sounding alarmist. Get the right questions to ask, where to find real alerts, and how to turn warnings into smart healthcare decisions.
Alcohol and Blood Thinners: What You Need to Know About Bleeding Risk and INR Changes
Posted by Desmond Carrington on 27/01/26
Alcohol can cause dangerous INR fluctuations in people taking warfarin, increasing bleeding risk. Learn how drinking affects blood thinners, what levels are safe, and what symptoms to watch for.
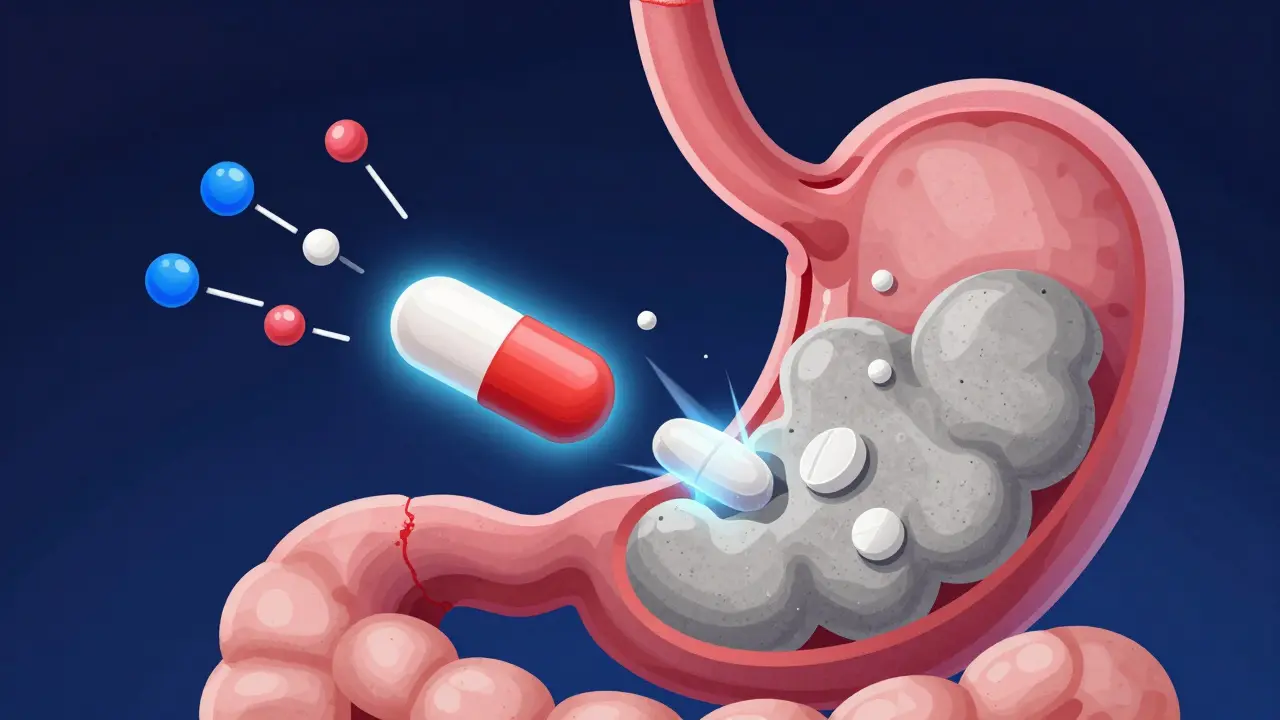
Antacids and Antibiotics: How to Time Your Doses to Avoid Treatment Failure
Posted by Desmond Carrington on 26/01/26
Taking antacids with antibiotics can reduce their effectiveness by up to 90%. Learn the exact timing rules for ciprofloxacin, doxycycline, and other common antibiotics to avoid treatment failure and antibiotic resistance.

Thalidomide and Teratogenic Medications: A History of Tragedy and Modern Safety Lessons
Posted by Desmond Carrington on 26/01/26
Thalidomide caused thousands of birth defects in the 1950s and 60s, leading to major drug safety reforms. Today, it's used under strict controls to treat cancer, but its history teaches vital lessons for pregnancy medication safety.

How to Talk to Patients About Switching to Generic NTI Drugs
Posted by Desmond Carrington on 23/01/26
When switching patients to generic NTI drugs like warfarin or levothyroxine, clear communication is critical. Learn what to say, when to monitor, and how to build trust to ensure safety and adherence.

How to Recognize Labeling Errors and Ask for Corrections in Medication Data
Posted by Desmond Carrington on 22/01/26
Learn how to spot and correct medication labeling errors in pharmacy systems to prevent dangerous mistakes. Practical steps for pharmacists and staff to ensure patient safety.
Statistical Analysis in BE Studies: How to Calculate Power and Sample Size Correctly
Posted by Desmond Carrington on 21/01/26
Learn how to correctly calculate power and sample size for bioequivalence studies to meet FDA and EMA requirements. Avoid common mistakes that lead to study failure.

SAMe and Antidepressants: What You Need to Know About Mood Effects and Interaction Risks
Posted by Desmond Carrington on 19/01/26
SAMe may help with mild depression, but combining it with antidepressants can trigger serotonin syndrome. Learn the risks, symptoms, and safe practices if you're considering this supplement.

Batch Release Testing: Final Checks Before Pharmaceutical Distribution
Posted by Desmond Carrington on 17/01/26
Batch release testing is the final, mandatory quality check that ensures every pharmaceutical batch meets safety and efficacy standards before reaching patients. It prevents recalls, protects public health, and involves rigorous lab testing and regulatory certification.

Beers Criteria: What Older Adults Should Know About Potentially Inappropriate Medications
Posted by Desmond Carrington on 16/01/26
The Beers Criteria identify potentially harmful medications for adults over 65. Learn which drugs to question, why they're risky, and how to use this tool safely with your doctor.

